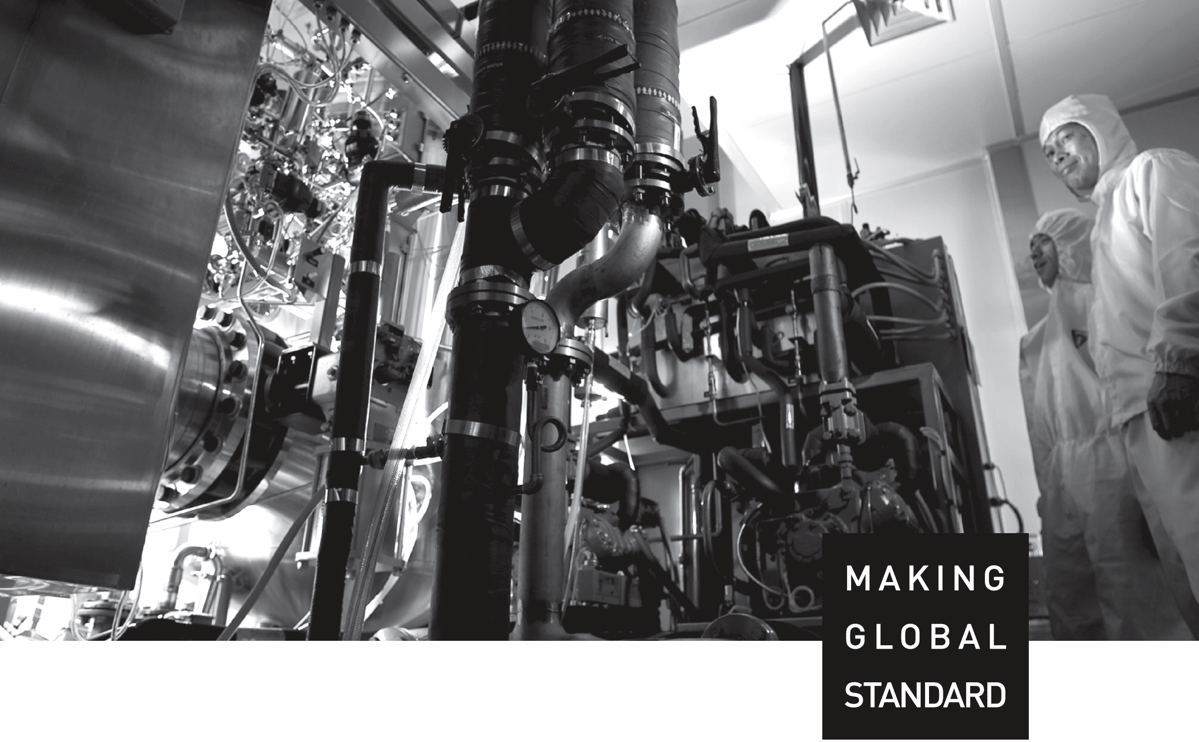
Company Introduction
Dongkook Pharmaceutical's spirit to step up to challenges results from our resilience
in overcoming uphill battles and passion in unexplored areas.
Since the construction of an API synthesis factory in 1985, we have done our best on the production
of excellent drugs through continuous study on products and expansion of investment.
1
Plant 1

Dongkook Pharmaceutical established an API synthesis factory in 1985 to extract and synthesize APIs of major products. Since then, we established Plant 1 in 1988 to open an era of KGMP in full scale. Plant 1 plays a role of the production of major active pharmaceutical ingredients to produce complete drugs and active pharmaceutical ingredients for exportation.
In Plant 1, major active pharmaceutical ingredients are manufactured such as propofol, streptokinase, and teicoplanin. Complete drugs out of such ingredients have been exported to not only the advanced pharmaceutical markets of Europe and Japan but also 40 countries in the world including Central & South America and India in Southwest Asia.
2
Plant 2

In 1992, Plant 2 was equipped with the mass production system of high-notch disposable syringe so called ‘Prefilled Syringe System’ for the first time in Asia. Since then, we established a Semi Auto Nest Type Prefilled System in 1997, an EFD Filling System of high viscosity, and a Prefilled Auto Filling System in 2010. Especially in 2007, in the field of syringe by GMP differentiation system conducted by the government, we received A-class to prove higher GMP management standard.
3
Plant 3


High-Notch Production & Quality Control System
Plant 3 has been manufacturing pills, capsules, ointments, and injections. Types of injections manufactured are SVP, LVP, and lyophilized injectable drugs. The Plant 3 that manufactures high quality complete drugs has received the excellent assessment of A-Class in the differentiation evaluation conducted by the government in 2008. We conducted productivity improvement and GMP upgrade in 2009 through the expansion and remodeling of previous facilities.
SVPSmall Volume Parentral
Representing product is a Pofol Injection which acquired KT Certificate in May 1996. Later, through the expanded installation of major facilities, currently the production is possible up to 750L, which was only 90L in 1998.
LVPLarge Volume Parentral
Representing product is a Pamiray Injection. We introduced a Pamiray production line in 1999 and an automatic filling line in 2005 to manufacture the best quality contrast medium.
Lyophilized injectable Drugs
Representing products are CMO products for Japanese customers, Teicon injection, Durakinase injection, and Lorelin Depot injection. Since 1996, we have introduced freeze drier, vial filling machine, and vial washing machine. Based on such introduction, we have accomplished the increase in production. Especially, the Lorelin Depot injection is the first Gn-RHa product developed by pure domestic technology and has acquired patents from several countries in the world.
4
Natural Product Extraction Factory

Dongkook Pharmaceutical decided to expand the facilities when the size of APIs synthesis of natural products had been increased largely out of the increase in cosmetics products of Madeca Cream and general drug of Insadol Plus and started a new construction at Plant 1 in early 2018. At the end of August in 2018, the new plant was completed with the size of 3,824.7m²(1,157 pyeong) and the high-tech GMP facility and named as ‘Natural Product Extraction Factory’.
By such expansion, we established a core manufacturing facility to produce various natural product materials of Dongkook Pharmaceutical such as Black Cohosh Extract and Magnolia Extract including ETIZM and TECA; and to solidity the position as a leading company in the production field of ‘Natural Herb Medicine’ and prepare the basis for the exportation of natural herb APIs.
Dongkook Pharmaceutical will expand the production line for prefilled injections at the Plant 2 and the production line for Pofol at the Plant 3 and continuously expand the production infrastructure through the facility investment in the field of capsules.
- History of Factories
- 1985. 04APIs factory completed at Hoejuk-ri, Manseung-myeon, Jincheon-gun, Chungcheongbuk-do
- 1988. 04K.G.M.P factory (Plant 1) completed at Hoejuk-ri, Manseung-myeon, Jincheon-gun, Chungcheongbuk-do
- 1989. 05Established a Central R&D Center of Dongkook Pharmaceutical Co., Ltd and acquired the certificate of the government
- 1991. 03Completed an injection factory (Plant 2), established the first prefilled syringe system in Korea
- 1991. 12Acquired K.G.M.P for Plant 1
- 1992. 07Acquired K.G.M.P for Plant 2 (Prefilled Syringe System)
- 1993. 07Completed Plant 3
- 1994. 07Acquired K.G.M.P for Plant 3
- 2001Expanded the production line of Plant 1
- 2004. 09Expanded Plant 3 with a fermentation factory (about 1,200 pyeong)
- 2010. 10Expanded Plant 2
- 2011. 04Newly built an automated storage for finished drugs (2,000 Cells)
- 2010. 10Expanded Plant 3 second time (About 3,200 pyeong)
- 2018. 09Constructed a dedicated factory for natural product extraction